inSight is a comprehensive integrated suite of applications designed to empower researchers with accurate and timely information. The wound assessment and documentation are streamlined with single image capture and synced to a secure HIPAA, GDPR, and 21 CFR Part 11 compliant environment.
inSight for Wound Care Research
Clinical Support Throughout the Study Life Cycle
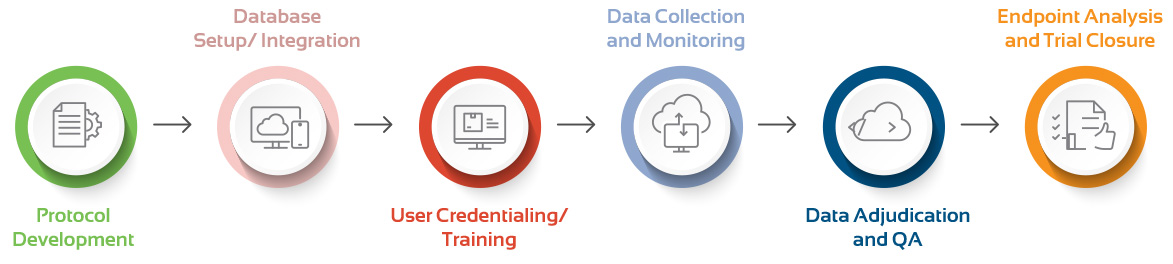
Pre-study Activities
- Create a unified integrated research experience. Leverage emerging data and clinical evidence to develop study protocol, imaging charter, and adjudication manual.
Study Built & Configuration
- Build and configure database with ease and efficiency. Customize clinical workflow and integrate seamlessly with your EDC.
Project Management
- Dedicated project manager to ensure timely start and project maintenance. De-risk your clinical study with highest quality standards, quality assurance and control procedures.
Data Analytics & Insights
- Analyze data and uncover insights in real-time. Curate data sets, augment clinical studies with real-world evidence.
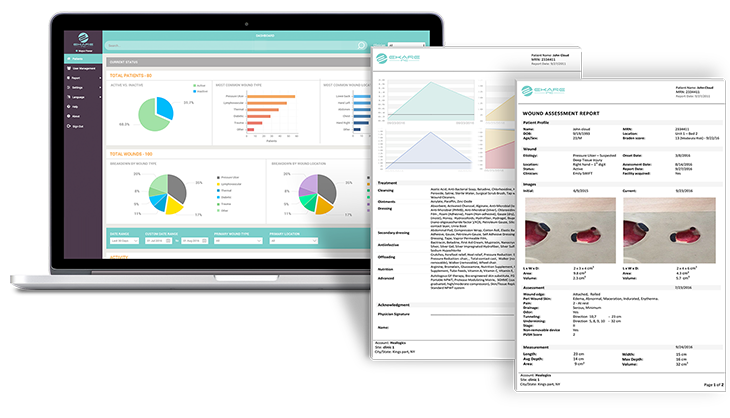
Robust Data Collection
eKare inSight yields high quality, consistent data. The system uses various quality assurance methods to help ensure all images collected produce accurate data.
Cross-Regulatory Compliance for Data Integrity and Security
inSight® has built-in measures for privacy, data encryption and security aligned with HIPAA and GDPR. Features include comprehensive user management, role-based access control, automatic user privilege assignment and revocation. The platform offers multi-layered protection for service availability, backup, and recovery.














