There continues to be a great need for advanced therapies in wound care, however, clinical trials evaluating cellular and tissue-based products (CTPs) often fail to include the population that will most likely benefit from it, reducing the value of data for clinical practice. Another negative consequence is that payers can deny coverage. Use of real-world data will be critical for assessing effectiveness and establishing benefit to actual patients. In this commentary, Dr. Fife dives deep to the heart of the issue, sharing her personal experience as a clinical trial investigator and a practitioner. -Raphael Yaakov, MS
Why Payers May Stop Covering Some Advanced Therapeutics for Hard to Heal Wounds
by Caroline E. Fife, MD
I am worried that payers are going to stop paying for treatments like cellular and/or tissue-based products (CTPs), previously called “skin substitutes,” unless the manufacturers begin performing clinical trials that are relevant to actual patients. In their defense, the manufacturers design these trials to satisfy the Food and Drug Administration (FDA). The best way to explain the problem is to tell you a story.
How I started (and why I stopped) being a clinical trial investigator
In 1997, I was a young faculty member at the University of Texas Health Science Center in Houston. I had been running a “wound center” for 7 years. I was one of many clinical investigators who participated in the clinical trials evaluating Becaplermin (REGRANEX®) gel and some months later, Apligraf® (a CTP) in the treatment of diabetic foot ulcers (DFUs). Both studies were randomized controlled trials (RCTs) and enrolled mostly Wagner I DFUs. In truth, the protocols said they also enrolled Wagner 2 DFUs, as long as there was no exposed tendon, joint capsule or bone (criteria which actually excluded most Wagner 2 DFUs). Although I had a very busy wound center in 1997 (the only wound center in the vast Texas Medical Center), I had to advertise in the local newspaper for clinical trial subjects. I couldn’t enroll any actual wound center patients because both RCTs excluded nearly every medical condition typical to patients with non-healing wounds (see Table 1 below). Some years later, Dr. Marissa Carter and I published a study comparing the exclusions in hundreds of wound healing trials with the prevalence rate of those conditions in actual patients. We demonstrated that about 75% of wound center patients could not have participated in the trials that brought novel advanced therapeutics to market. In fact, these RCTs had so many exclusion criteria they enrolled subjects who were healthier than the average person on the street.
Here are some photos I dug up of actual subjects I enrolled in the 2 aforementioned trials, compared to a typical patient in my clinic around the same time (Fig 1):
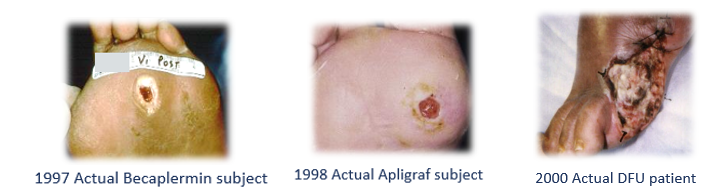
In 1998, I was excited to have Apligraf finally on the market. In fact, thanks to Apligraf and Negative Pressure Wound Therapy (NPWT) which I had only been using for a year, I was able to heal a woman in her 70’s with a horrible leg ulcer that had been present for more than 6 months (Fig 2). She had rheumatoid arthritis on prednisone, peripheral arterial disease and diabetes. It’s likely that this was an inflammatory or vasculitic ulcer, but the biopsy did not show that. It looked like CTPs were going to revolutionize wound care! But there was a big problem brewing…
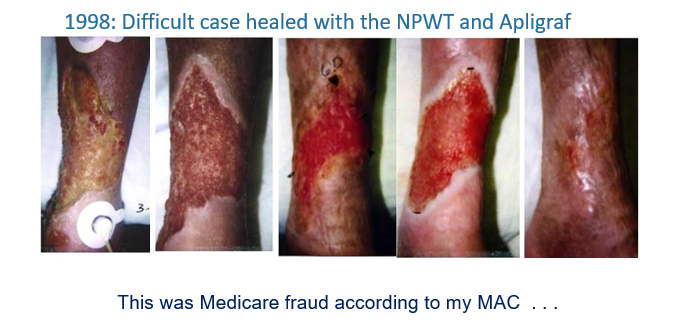
Read the fine print on the coverage policy
In 1999, my Medicare “contractor” (which at the time was trailblazer) wrote a coverage policy for “skin substitutes” which said that these products, “… must not be provided to patients with: uncontrolled diabetes, vasculitis, rheumatoid arthritis, ongoing use of corticosteroids, or immunosuppressants.” I had just healed a patient who had 3 of those excluded conditions. This meant that if I was audited by Medicare, I’d have to pay the money back for the Apligraf I applied, because I failed to comply with Medicare coverage policy. I recognized the list of exclusions because I had been an investigator in the RCT. The coverage policy had been crafted using the exclusion criteria of the clinical trial.
As a result, by 2002, I refused to participate in any more wound healing RCTs. There was nothing unscientific about these clinical trials. In fact, they were designed exactly the way that the FDA wanted them to be designed. There was certainly nothing unethical about them. However, in my mind, it was just WRONG to perform trials with subjects who bore no resemblance to actual patients if the payers were then going to refuse to allow us to use the products on the patients who needed them! We were spending millions of dollars to bring products to market so that payers could refuse to cover them using the irrefutable logic that we didn’t know if the products would work on real patients!
When I started complaining about this issue two decades ago, no one was interested. However, now that some Medicare Administrative Carriers (MACs) have begun audits of CTPs and a few hospitals have paid back money for using them outside of the coverage policy, and now that some private payers are simply refusing to cover CTPs for many patients, there’s a sudden interest in “real-world data.” It has taken two decades but it’s finally time to reconcile these opposing forces.
The Real World vs. the Clinical Trial World
In 2017, we used a consortium of 6 clinics that participated in the US Wound Registry (USWR) to compare actual patients with DFUs to those enrolled in RCTs performed in those same clinics. The 6 clinics treated 2,634 DFUs and also enrolled 244 DFU subjects in 4 RCTs of CTPs.
Here’s how the real-world patients differed from subjects enrolled in CTP RCTs:
- 12.2% of real DFU patients had renal failure – a condition excluded from all RCTs
- Among real world patients, there were 4.3 DFUs per patient, but the RCTs enrolled only 1 ulcer per patient and didn’t even record how many there were present
- 43.6% of real-world patients had ulcers that were Wagner 3 or worse ulcers whereas RCTs enrolled Wagner 1 and 2 ulcers (as long as the Wagner 2 ulcers were not too bad)
- Among real patients, the initial DFU ulcer surface area was 3 times larger than ulcers in the RCTs
The Wound Healing Index (WHI) is a validated method of stratifying both the wound and the patient with the wound. It is a way of predicting healing likelihood and allows us to compare the outcome of patients who are equally sick. Currently, the WHI is integrated into Intellicure’s wound care EHR. According to the WHI, real-world DFUs were statistically much less likely to heal than those enrolled in the cellular product RCTs (68.6 vs. 88.1). In fact, the WHI confirmed that the subjects enrolled in the RCTs of cellular products bore no resemblance to the typical DFU patient. This makes RCT data virtually worthless for assessing the potential value of a CTP among real world patients.
The story was the same for venous leg ulcers (VLUs). Real VLU patients have multiple ulcers that are huge, the patients are more likely to be elderly, they take 4 or 5 times longer to heal than the duration of a typical RCT, many real world VLUs get worse during treatment, and co-morbid conditions that are excluded in RCTs are COMMONLY present in real world patients.
Is there a way forward?
The FDA wants RCTs and the endpoint they want is complete healing. Manufactures will not invest millions of dollars into clinical trials that fail to show a sufficient percentage of subjects with complete healing. That means the manufacturers have to design trials with subjects who do not resemble actual patients because nearly half of actual patients do not heal. Unfortunately, payers may deny coverage for CTPs based on the Kafkaesque logic that the products have not been tested in the real world. In other words, the FDA wants efficacy data which can only be obtained in an imaginary perfect world. The payers want effectiveness data which can only be obtained in the real world. Apparently, we are going to need both of these types of data, or advanced therapeutics like CTPs will just not be covered by payers.
We were going to talk about this conundrum in D.C. last spring at the Wound Care Evidence Summit, bringing together the FDA, Medicare and private payers, manufacturers and clinicians. This is not something we can do by Zoom. We will have to get into the same room. I hope we will have the chance to talk about it in the fall of 2021. Maybe one outcome of the pandemic will be a willingness to think differently about clinical trial design in the wake of a painful, global reality check. I don’t see a future for advanced wound treatment modalities unless some serious research problems are addressed.
Table 1. Exclusion Criteria for the Typical Wound Healing RCT (subjects could not be enrolled if the following were present):
- For DFU studies, no ulcers > Wagner Grade II
- Diabetes as a co-morbid condition for any study other than DFU
- Venous stasis except in VSU trials
- Alcohol/drug abuse
- Anticoagulant treatment
- Cellulitis or local wound infection
- Cancer or recent cancer treatment
- Collagen vascular disease/connective tissue disease
- Rheumatoid arthritis/autoimmune disease, any type
- Scleroderma/lupus, any autoimmune disease
- Charcot foot changes in DFU
- Corticosteroid treatment any reason
- Deep venous thrombosis/pulmonary embolus
- Gastrointestinal disease of any kind /any Liver disease/Hepatitis
- Renal impairement/ESRD/Renal dialysis/Renal transplant
- Any organ transplant
- In diabetics, HbA1c > 8-10
- Nutritional impairment/Albumin < 3.0 mg/dl
- Osteomyelitis
- Peripheral arterial disease
About the Author

Dr. Fife completed a Family Medicine residency at the University of Texas, Southwestern in Dallas followed by a two year Fellowship in Undersea and Hyperbaric Medicine at Duke University. Until 2013 she was a Professor of Medicine at the University of Texas Health Science Center, Houston where she initiated the Memorial Hermann Center for Wound Healing and Hyperbaric Medicine and the Lymphedema Center. She is now a Professor of Geriatrics at Baylor College of Medicine in Houston, and the Medical Director of the CHI St. Luke’s Wound Clinic in The Woodlands, Texas. She is also the Chief Medical Officer of Intellicure, Inc., a health information technology company, and the Executive Director of the U.S. Wound Registry, a non-profit organization recognized by CMS as a qualified clinical data registry. The USWR develops quality measures relevant to hyperbaric and wound management practitioners and helps those specialists meet the requirements of Medicare’s new Quality Payment Program. She has been a Certified Wound Specialist since 1998 and is a past president of the Undersea and Hyperbaric Medical Society. Past and present board activities include the Alliance of Wound Care Stakeholders (current co-chair), the American Academy of Wound Management, the Association for the Advancement of Wound Care and the American Professional Wound Care Association. She is the clinical editor of Today’s Wound Clinic, and has authored more than 100 peer reviewed articles and book chapters as well as editing 3 textbooks (the Textbook of Chronic Wound Care, Wound Care Practice, and Women and Pressure: Diving and Flying.). Her research contributions include altitude decompression studies that enabled the construction of the International Space Station by decreasing the time needed for oxygen pre-breathe as part of a NASA lead research consortium, the development of real time lymphatic imaging with Dr. Eva Sevick using near infrared technology, and more recently, the use of real world data for comparative effectiveness studies to understand what works best for patients with chronic wounds and ulcers.
